
|
Associate Professor Vimol Srisukh Department of Food Chemistry, Faculty of Pharmacy, Mahidol University |
|
| 145,785 View 2 hours ago | |
| Publication date: 2012-05-27 |
At your latest (routine) health check-up, you’ve just found out that you are diabetic. Your life won’t be the same again. Limitless choice of foods seems to be remote and countless teaspoonfuls of sugar in your morning coffee have now become only a dream. What choice of alternative sweeteners do you have? 
Group 1: sweeteners containing only fructose
Usually, fructose can be found naturally in fruits. As in other carbohydrates, it contains 4 Kcal/g of energy. But since fructose is 1.3 times sweeter than sugar, it is only needed in smaller amounts to obtain the same level of sweetness. The advantage is that fructose is absorbed more slowly than glucose, producing therefore a lower insulin response than in the case of sucrose. Nevertheless, consuming high amounts of fructose can lead to high levels of triglyceride and cause gastro-intestinal symptoms in some susceptible individuals. Thus, according to some report, fructose is not recommended for people with diabetes, because of concern for increased blood lipid levels.
There is only one sweetener containing fructose available in local supermarkets. 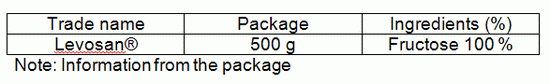
Note: Information from the package
Group 2: sweeteners containing Aspartame
Aspartame is an intense sweetener which is 160-200 times sweeter than sucrose. It has been approved, by the USFDA, as a general-purpose sweetener. Although Aspartame provides 4 Kcal/g of energy (same as sucrose), its sweetness is much higher, so only a small amount of Aspartame is need to achieve the desired level of sweetness, and thus generates much less energy.
The drawback to the use of Aspartame is its bitter taste when consumed at high dosage; nor is it heat stable. The latter property limits its use in normal cooking and baking. Aspartame is not stable in beverages if stored for long periods of time. When ingested, Aspartame is hydrolyzed into aspartic acid, phenylalanine, and methanol. Because Aspartame contains phenylalanine, its use is controlled to prevent individuals with the rare disorder of phenylketonuria from ingesting this substance. Phenylketonuria is a genetic deficiency of a particular enzyme, phenylalanine hydroxylase, resulting in the accumulation of phenylalanine and its metabolites. This may produce a syndrome of mental deficiency, epileptic seizure, reduced pigmentation, muscular hypertonicity, hyperkinesias (excessive movements), EEG (brain wave) abnormalities and growth retardation. On packages of sweeteners which contain Aspartame, you will notice warning labels stating that this sweetener should not be used for people with phenylketonuria. In addition, this is a sweetener about which people have periodic safety concerns, since some animal studies have shown that it can cause cancer at levels close to the Acceptable Daily Intake (ADI)* in human. The US Food and Drug Administration and European Food Safety Authority have both set an acceptable daily dose of 50 mg/kg body weight/day which decreases to 40 mg in the case of phenylketonuria. Aspartame has also been linked to various neuropsychiatric disorders, including panic attacks, mood changes, visual hallucinations, manic episodes and isolated dizziness. It is also linked to headaches in some individuals. On the other hand, there are reports about the safety of Aspartame stating that Aspartame is safe at present levels of consumption. With this caution in people’s mind, manufacturers of other alternative sweeteners have chosen to emphasize the point by highlighting on the label that the contents contain “No Aspartame”.
From our survey, sweeteners in this category are available commercially, from local supermarkets, in both powder and tablet form. 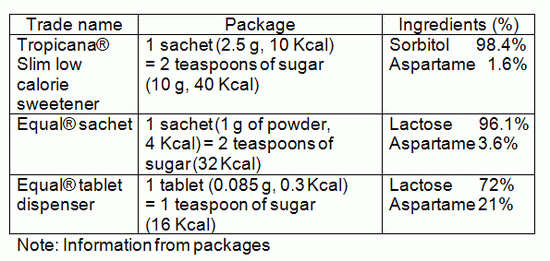
Note: Information from the package
Since only a small amount of Aspartame is required (because of its intense sweetness), other ingredients, such as Sorbitol and lactose, are needed as fillers for the sachets. In addition to being fillers, these sweeteners are included in order to replace some functional properties (e.g. viscosity) of sucrose. The trend is to blend high-intensity sweeteners with other nonnutritive and nutritive sweeteners to create new sweet taste profiles. Sorbitol is a sweetener from the Sugar alcohol group, called Polyols. Sorbitol is only 60% as sweet as sucrose and provides only 2.6 Kcal/g. The advantage of Sorbitol is that it is absorbed incompletely, and at a much slower rate than sucrose, so it will not cause a rapid elevation of insulin response, as in the case of glucose or sucrose. Thus it is a good choice as an alternative sweetener for diabetics in their own right. Nonetheless, consuming Sorbitol in high quantities can result in diarrhoea (Sorbitol, at more than 50 g/day). Lactose is used as another filler to increase the package content. Lactose is only 0.2 time as sweet as sucrose, thus it is not intended to be the main sweetener in the product. Lactose can also be digested into glucose and galactose which are both actively absorbed. People with a lactose intolerance problem might have some gastro-intestinal symptoms such as flatulence and diarrhoea, when consuming high amount of lactose (more than 3-5 g or higher).
(*ADI = the estimated amount (usually in milligrams) per kilogram of body weight that a person can safely consume on average every day over a lifetime without risk)
Group 3: sweeteners containing Sucralose
Sucralose is an intense sweetener which is 600 times sweeter than sucrose. It is approved by the USFDA for use as a general-purpose sweetener. Sucralose provides a high quality of sweetness, similar to sucrose but with no bitter taste. It has excellent stability in a wide range of processed foods and beverages. It is heat stable at cooking and baking temperatures. Since Sucralose is a relatively new intense sweetener, compared to the rest of intense sweeteners, monitoring the long-term studies of Sucralose is suggested. The ADI of Sucralose is 15 mg/kg body weight/day.
From our survey, quite a few products are available in local supermarkets. 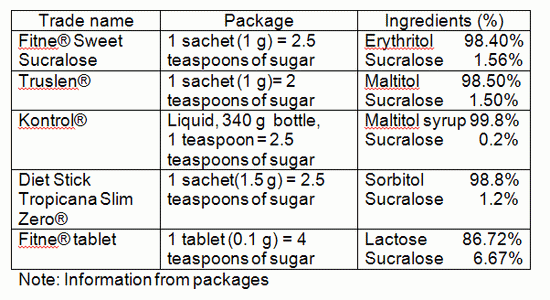
Note: Information from the package
Since Sucralose is very sweet, only small amounts are needed to produce the same sweetness as sucrose. Similar to other intense sweeteners, additional ingredients are needed as fillers for the sachet. Lactose is used as a filler in one product (check the precaution in Group 2). Other fillers include sweeteners from the Sugar alcohol group, called Polyols. Generally, most of the compounds in this group are less sweet than sucrose e.g. Maltitol (90-95% as sweet as sucrose, 2.1 Kcal/g), Sorbitol (60% as sweet as sucrose, 2.6 Kcal/g), Erythritol (70% as sweet as sucrose, 0.2 Kcal/g), etc. The advantage of these sweeteners is that they absorb incompletely and at a much slower rate than sucrose, so they will not cause a rapid elevation of insulin response, as in the case of glucose or sucrose. Thus they are a good choice as an alternative sweetener for diabetics in their own right. Nonetheless, consuming these sweeteners in high quantities can result in diarrhoea (e.g. Sorbitol, at more than 50 g/day).
Group 4: sweeteners containing Acesulfame-K, in combination with Aspartame
Acesulfame-K is an intense sweetener which is 200 times sweeter than sucrose. It is approved by the USFDA for use as a general-purpose sweetener. Acesulfame-K’s sweetness can be perceived rapidly without any unpleasant delay. But a bitter taste is sometime detectable at high concentrations. The difference between Acesulfame-K and Aspartame is that the former provides 0 Kcal/g of energy. Acesulfame-K can withstand high cooking and baking temperatures. The heat stability differentiates Acesulfame-K from Aspartame. The Acceptable Daily Intake (ADI) for Acesulfame-K is 15 mg/kg body weight/day. Acesulfame-K is often used in combination with Aspartame, in the proportion of 1:1, which results in synergism, thereby, reducing the concentration of each sweetener down to a level that will not cause a bitter taste in the product.
There is one sweetener containing Acesulfame-K in combination with Aspartame at local supermarkets. 
Note: Information from the package
Since Acesulfame-K and Aspartame, respectively, are 200 times and 160-220 times sweeter than sugar, only small amounts of the sweeteners are needed, so maltodextrin is usually added as a bulking agent (a filler). Maltodextrin, if not being a resistant maltodextrin (a soluble fiber), has a Glycemic Index of 106-136 (the GIs of sugar and glucose are 65 and 100, respectively).
In addition, another sweetener which contain Acesulfame-K in combination with Aspartame is categorized in Group 6.
Group 5: sweeteners containing stevioside
This group of sweeteners contains natural compounds. Leaves from the plant, Stevia rebaudiana Bertoni, mainly consist of Steviol glycosides. The major constituents are Stevioside and Rebaudioside A. Stevioside is a compound which is 300 times sweeter than sucrose; Rebaudioside A is even sweeter, at 450 times. The leaves, as well as the pure Stevioside extract, can be used in its natural state or cooked, and are thermostable at temperatures of up to 200?C. It is a sweetener with low caloric value. According to analysis, dried leaves of Stevia contain 2.7 Kcal/g. Stevia sweeteners, extracted from the leaves, are commercially available in Japan, Korea, China, and South East Asia. In the U.S.A., the powdered leaves and their extracts are used only as a dietary supplement. As for purified Rebaudioside A, the USFDA states that it can be considered GRAS (Generally Recognized As Safe). On the other hand, steviol glycosides have not been approved by the European Commission, who maintain safety concerns. A temporary ADI of 0-4 mg/kg body weight of Steviol glycoside, an equivalent of 0-10 mg/kg body weight of stevioside, has been suggested. If not ingested at a high level, steviol glycosides are still considered safe.
From our survey, there is only one commercially available product. 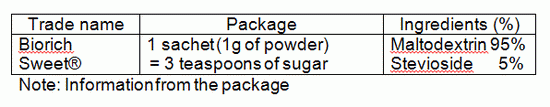
Note: Information from the package
Because of its intense sweetness, only small amounts of stevioside are packed in each sachet, so maltodextrin is usually added as a filler (check the precaution in Group 3).
Group 6: sweeteners containing sucrose and/or dextrose (glucose)
Although this group of sweeteners contains intense sweeteners, such as Sucralose, Acesulfame-K, and Aspartame (check the precaution for Aspartame in Group 2) and sweeteners in the Sugar alcohol group, they still contain sucrose or dextrose at high percentages (more than 90%). Both sucrose and dextrose can induce rapid insulin response. Since sweeteners in this group contain natural sugars, they have the advantage of providing familiar sweet taste. And since the products contain at least one intense sweetener, the sweeteners can provide the same level of sweetness with reduced amounts of sucrose or dextrose. At a quick glance, the sweeteners’ main target group is probably not for diabetics, but they are targeted more at consumers who wish to control their calories or lose body weight and this marketing message is backed up by the “slim” sachets used for packaging.
The sweeteners available in local supermarkets are as follows: 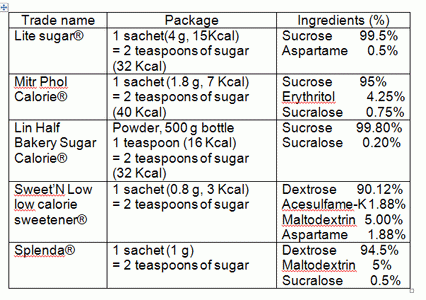
Note: Information from the package
Conclusion
When selecting alternative sweeteners (as a substitute for sugar), diabetics tend to consider “taste” as a primary consideration since they still want to live normal lives. Sweeteners which provide normal coffee flavor tend to be more popular choices than those which impart a strange flavour. Nonetheless, sucrose is also found in other normal foods, which is why care should always be taken when selecting dishes for any meal.
Note: an educational article about foods and nutrition by Associate Professor Vimol Srisukh, Department of Food Chemistry. Edited by N.&V.P. of U.K.
Reference
- American Dietetic Association. Position of the American Dietetic Association: use of nutritive and nonnutritive sweeteners. J Am Diet Assoc 2004; 104:225-75.
- Association AD. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care 2003; 26:51S-61S.
- Belpoggi F, Soffritti M, Padovani M, Degli Esposti D, Lauriola M, Minardi F. Results of long-term carcinogenicity bioassay on Sprague-Dawley rats exposed to aspartame administered in feed. Ann NY Acd Sci 2006; 1076:559-77.
- Butchko HH, Stargel WW, Comer CP, Mayhew DA, Benninger C, Blackburn GL, de Sonneville LMJ, Geha RS, Hertelendy Z, Koestner A, Leon AS, Liepa GU, McMartin KE, Mendenhall CL. Aspartame: review of safety. Regulatory Toxicology and Pharmacology 2002; 35:S1-93.
- Lemus-Mondaca R, Vega-Galvez A, Zura-Bravo L, Ah-Hen K. Stevis rebaudiana Bertoni, source of a high-potency natural sweetener: a comprehensive review on the biochemical nutritional and functional aspects. Food Chem 2012; 132:1121-32.
- McKittrick M. Mysterious maltodextrin. PCOSA Today Newsletter Spring 2009 Issue. (retrieved on April 5, 2012 at http://www.pcosupport.org/newsletter/articles/article 121008-3.php)
- Magnuson BA, Burdock GA, Doull J, Kroes RM, Marsh GM, Pariza MW, Spencer PS, Waddell WJ, Walker R, Williams GM. Aspartame: a safety evaluation based on current use levels, regulations, and toxicological and epidemiological studies. Crit Rev in Toxicol 2007; 37:629-727.
- Puri M, Sharma D, Tiwari AK. Downstream processing of stevioside and its potential applications. Biotech Adv 2011; 29:781-91.
- Soffritti M, Belpoggi F, Degli Esposti D, Lambertini L. Aspartame induces lymphomas and leukemias in rats. Eur J Oncol 2005; 10:107-16.
- Soffritti M, Belpoggi F, Degli Esposti D, Lambertini L, Tibaldi E, Rigano A. First experimental demonstration of the multipotential carcinogenic effects of aspartame administration in the feed to Sprague-Dawley rats. Environ Health Perspect 2006; 114:379-85.
- Soffritti M, Belpoggi F, Tibaldi E, Degli Esposti D, Lauriola M. Life-span exposure to low doses of Aspartame beginning during prenatal life increases cancer effects in rats. Environ Health Perspect 2007; 115(9):1293-7.
- Ursino MG, Pouzzi E, Caramella C, De Ponti F. Excipients in medicinal products used in gastroenterology as a possible cause of side effects. Regulatory Toxicology and Pharmacology 2011; 60:93-105.
Recommended articles
|
|
Preservatives in sausages, ham, and bologna 1 minutes ago |

|
Young Coconut Water: The functional drink from nature 8 minutes ago |

|
Thai Traditional Medicine Theory in Relation to Seasons Part 3 1 hours ago |

|
Top 10 Selenium-Rich Foods & Health Benefit 2 hours ago |

|
Top 10 Potassium-Rich Foods & Health Benefit 2 hours ago |
|
Thai Traditional Medicine Theory in Relation to Seasons Part 1 2 hours ago |
|
AMD.. what to eat to delay it? 2 hours ago |

|
Stevia: Natural sweetness...sweetener of choice 2 hours ago |

|
STOP SMOKING with Nicotine Replacement Therapy 2 hours ago |

|
Health effects of plant-based diets 3 hours ago |
